orange book pharmacy ab rating
College of Pharmacy and Health Sciences. Orange book pharmacy ab rating SHARE.

Growing Up With Video Games In The 70 S And 80 S I Was Well Aware Of Pixels When Video Games First Started Out The Screen Re Pixel Art Art Activities Pixel
I am been looking at the orange book and still not sure what is the best way to look up that information.

. Search approved drug products by active ingredient proprietary name. A de o co aacs 55 o. Must a drug be rated AB in FDAs Orange Book to be used in product selection in North Carolina.
According to the FDA two products are considered to be bioequivalent if the 90 clearance CI of the relative mean Cmax AUC 0 - t and AUC 0 - of the generic drug to the brand - name. Also the annual Orange Book Edition Appendices A B and C in PDF format are updated quarterly. CDR Kendra Stewart RPh PharmD.
Since February 2005 we have been providing daily Electronic Orange Book EOB product information for new generic drug approvals. For example if I have a script for Cartia XT and my pharmacy have 5 differents diltiazem ER how do I use the orange book to see which one is AB rate. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been approved in applications under section 505 of the FDC Act because these products are no longer listed drugs see section 7002 e 4 of the Biologics Price.
Basics in drug approval process with reference to the Orange Book Presented by. Applying the Ratings Code to Antihypertensive Agents. Orange book pharmacy ab rating Friday March 25 2022 Edit According to the FDA two products are considered to be bioequivalent if the 90 clearance CI of the relative mean Cmax AUC 0 - t and AUC 0 - of the generic drug to the brand - name.
Professor Department of Pharmacy Practice. Monday February 28 2022. 200833 6 Generic Drug Review30-34.
The electronic availability of the Orange Book brings this valuable tool to the web for healthcare. With respect to the dilemma concerning Cardizem CD noted earlier a search of the Orange Book revealed that Cardizem CD 240 mg is rated AB3. The North Carolina Product Selection Law does not refer to the Orange Book rating published by the Food and Drug Administration.
Vivian BS Pharm JD. When the first generic Concerta products came to market late in 2013 they were Orange Book AB rated to the brand name. Preface to Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book provides info on how the book came to be relevant terms and codes user responsibilities and more.
Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use. The electronic Orange Book provides guidance that is beneficial for pharmacy personnel to review. A guide to community pharmacist.
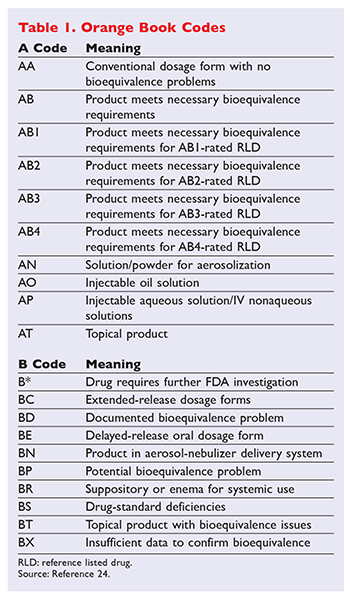
Drugs rated as AB1 are bioequivalent and pharmaceutically equivalent to each other as are drugs that are AB2-rated and so on. As oae boo ad ab as o aaceca d odcs. How often is the Orange Book updated.
As such it is essential that pharmacists practicing in New York State have a thorough understanding of the Orange Book and the leading role it plays in ensuring the safety and effectiveness of the drug products dispensed in. Office of Generic Drugs Policy Center for Drug Evaluation Research US. Pharmacists should be familiar with the legal issues involved when they dispense a drug manufactured by a.
As pharmacists are aware in recent weeks the Food and Drug Administration FDA changed the Orange Book equivalency rating of extended release methylphenidate products manufactured by Mallinkrodt and Kudco from AB to BX due to concerns about bioavailability equivalency with Janssen Pharmaceuticals Concerta product. The Orange Book uses Therapeutic Equivalence codes TE codes a short series of letters and sometimes numbers eg AB AB2 BX to categorize drugs based upon their assessed equivalency. 1 Need of the Orange Book Definition Introduction to 2 History 3 the Orange Book Objectives 4 3 Contents of the Orange Book 5 18 4 Cumulative Supplement 19 5.
A guide to community pharmacist. 1015406mojbb20150100013 Table 1 Summary of FDAs Orange Book Therapeutic Equivalence Codes Code Interpretation. That rating can be useful in determining what is used in product selection but it is not necessarily determinative.
Food and Drug Administration. What rating must a generic have to be considered clinically bioequivalent to the brand name drug. This book Approved Drug Products With Therapeutic Equivalence Evaluations also known as the orange book because it has a bright orange cover is available both in print and online to anyone but is intended for use by.
The Orange Book has long been a reliable resource for information about FDA-approved drugs. Generic Drugs and The Orange Book. Home ab book orange wallpaper orange book pharmacy ab rating.
The orange book is a list of generic drugs approved by FDA. Rucha Pathak Roll No. Approved Drug Products with Therapeutic Equivalence Evaluations.
Search the Orange Book Database. For more information on the Orange Book including its history see the Orange Book Preface. This means they were classified as bioequivalent by the FDA and therefore were substitutable with one another.
FDAs orange book and ab ratings of pharmaceutical drug products. FDAs orange book and ab ratings of pharmaceutical drug products. Orange Book in choosing drugs for generic substitution.
What are the AB rated generics for brand Concerta. An A designation means that the FDA considers the drug to be the therapeutic. The first letter indicates that the FDA has either concluded a generic formulation is therapeutically equivalent to the reference drug an A Code rating or that the compared drugs.
The first letter -- A or B -- indicates whether the drug is therapeutically equivalent to other pharmaceutically equivalent products. The orange book is available in electronic format Electronic Orange Book to provide access to information such as. Thus generic products rated AB3 such as Apotexs generic are suitable for substitution.
Every drug listed in the Orange Book has a 2-letter code. A book published by the FDA each year and updated periodically also provides guidance about which drugs are interchangeable.
Insights Into Effective Generic Substitution

Organic Raw Unfiltered Pure Honey 12oz Good Gather Unfiltered Honey Pure Honey Honey

Special Rates On The Secret Garden At Otres Beach Hotel Sihanoukville Read Real Guest Reviews Find Great Deals Beach Hotels Secret Garden Beachfront Property
Minerals Free Full Text Exceptional Multi Stage Mineralization Of Secondary Minerals In Cavities Of Flood Basalts From The Deccan Volcanic Province India Html

Kvs设计 On Behance Neon Signs Design Mood Boards
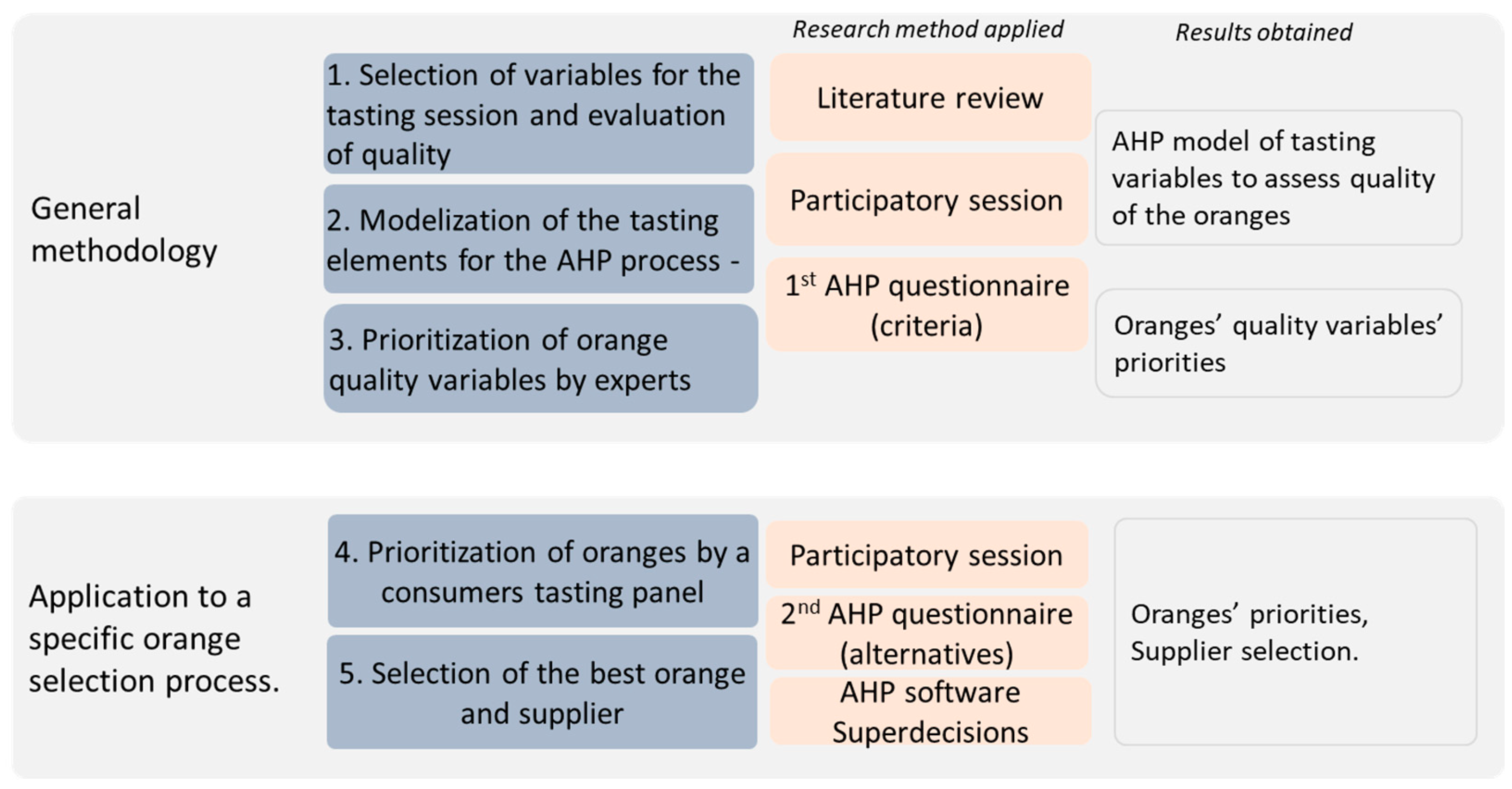
Ijerph Free Full Text Proposal Of A New Orange Selection Process Using Sensory Panels And Ahp Html

Abdominal Compartment Syndrome Powerpoint Template Powerpoint Templates Free Powerpoint Templates Download Powerpoint Template Free
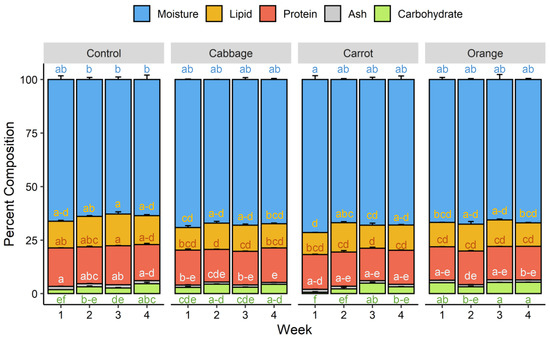
Foods Free Full Text Growth Performance And Nutrient Composition Of Mealworms Tenebrio Molitor Fed On Fresh Plant Materials Supplemented Diets Html

Comparison Chart Powerpoint Template And Keynote Slide Comparison Chart Powerpoint Template A Powerpoint Chart Templates Powerpoint Charts Powerpoint Templates

A Peaceful Sketch Tea Drawings Canvas Happy Drawing

Nativa Turbovite Capsules 30 S Book Cover Capsule Mindfulness

Pin By Mary Martin On Magnificent Drums And Drummers Vinyl Record Clock Drummer Gifts Music Clock

Anatomy Of The Eye Diagrams For Coloring Labeling With Reference And Summary Eye Anatomy Diagram Human Eye Diagram Eye Anatomy

12 Hour Shift Funny Nurse Quotes Night Shift Humor Nurse Quotes


